Cowden Syndrome: Cobblestone Tongue and Cancer Prevention Insights
A Spanish woman in her 60s arriving with persistent dry mouth and a baffling oral landscape ultimately revealed a diagnosis of Cowden syndrome. This rare, autosomal dominant disorder, rooted in germline PTEN mutations, predisposes patients to multiple hamartomas and carcinoma risks, demanding rigorous lifelong surveillance. Here, we explore the clinical findings, molecular underpinnings, and emerging management strategies.
Case Presentation
The patient, aged 62, presented to a dermatology clinic in Barcelona after three months of xerostomia and mucosal discomfort. Her history included invasive ductal carcinoma of the breast at 48, papillary thyroid carcinoma at 55, and a resected colon adenoma. A positive family history of breast and thyroid neoplasms suggested a hereditary cancer predisposition.
Clinical Findings and Diagnosis
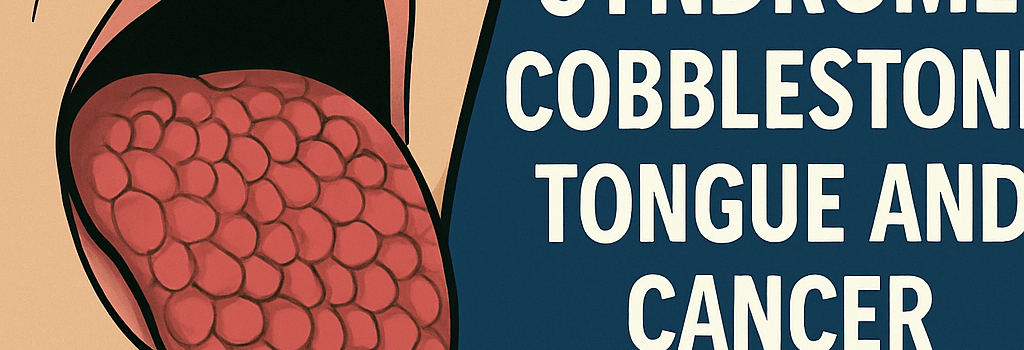
Oral Manifestations and Cobblestone Tongue
Examination revealed numerous 1–3 mm papillomatous papules on the dorsal tongue, yielding a cobblestone texture. These flesh-colored lesions on the anterior two thirds of the tongue are pathognomonic for Cowden syndrome and often precede malignant transformations by decades.
Genetic Confirmation
Targeted next-generation sequencing identified a heterozygous nonsense variant in exon 5 of PTEN, resulting in truncation of the phosphatase domain. Variant classification followed ACMG guidelines, confirming pathogenicity. No evidence of somatic mosaicism was detected in buccal or blood samples.
Genetic Mechanisms and Pathway Dysregulation
PTEN encodes a dual-specificity phosphatase that converts PIP3 to PIP2, restraining the PI3K/AKT/mTOR signaling axis. Loss of PTEN function leads to PIP3 accumulation, constitutive AKT activation, and enhanced mTORC1-mediated protein synthesis and cell proliferation. Cryo-EM studies at 3.3 Å resolution have detailed conformational shifts in the PTEN C2 domain that disrupt membrane binding, unveiling allosteric pockets for drug design. Over 1 000 germline PTEN variants are cataloged in ClinVar, with hotspot mutations clustering in the WPD loop and TI loop, critical for phosphatase activity.
Molecular Diagnostics and Variant Interpretation
Comprehensive genetic panels for hereditary cancer syndromes now include full PTEN coding region coverage, splice site analysis, and promoter screening. Multiplex ligation-dependent probe amplification (MLPA) detects large deletions or duplications not captured by NGS. Variant pathogenicity relies on functional assays, in silico predictions of protein stability, and population frequency data from gnomAD. RNA sequencing can reveal aberrant splicing events driven by intronic variants.
Advances in PTEN-Targeted Therapies
Although no agents restore germline PTEN, mTOR inhibitors such as everolimus have shown partial regression of mucocutaneous hamartomas in phase II trials. A recent multicenter phase III study of the AKT inhibitor ipatasertib plus fulvestrant reported a median progression-free survival improvement in PTEN-deficient breast cancer cohorts. Dual PI3K/mTOR inhibitors and selective AKT1 inhibitors are in phase I/II development. Preclinical CRISPR base editing approaches successfully corrected PTEN loss in patient-derived cell lines. Combination regimens targeting compensatory feedback via PI3K-delta and HSP90 inhibitors are being evaluated in xenograft models. In May 2025, the FDA granted breakthrough device designation to a liquid biopsy assay quantifying PTEN mRNA isoforms for early tumor detection in Cowden syndrome carriers.
Clinical Recommendations and Surveillance Protocols
- Annual thyroid ultrasound beginning at age 7 to 10 to detect cysts or nodules early
- Clinical breast exam every 6 months from age 25 and annual breast MRI starting at 30
- Colonoscopy every 5 years beginning at 35 or 10 years before the youngest familial colorectal cancer
- Dermatologic and mucosal evaluation every 6 to 12 months for new hamartomas or malignant changes
- Endometrial cancer surveillance with transvaginal ultrasound or biopsy starting at age 30 to 35
Future Directions and Research
Emerging modalities include liquid biopsy for circulating tumor DNA sequences capturing PTEN loss of heterozygosity and single-cell RNA sequencing to map transcriptomic heterogeneity in hamartomas. Machine learning models trained on multiparametric imaging and genomics aim to predict malignant transformation risk. International registries facilitate genotype-phenotype correlation and natural history studies, guiding personalized interventions. Integration of AI-driven predictive analytics with clinical workflows may shorten diagnostic delays for rare syndrome patients.
Dr Maria Fernandez, a clinical geneticist at the University of Barcelona, observes that integrating genomic data with AI-driven predictive models could revolutionize surveillance and reduce the diagnostic odyssey for Cowden syndrome patients.